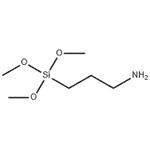
3-Aminopropyltrimethoxysilane: Self-Assembly and Functional Applications
Sep 4,2025
![]()
![]() 3-Aminopropyltrimeth-Oxysilane' Self-Assembled Effect
3-Aminopropyltrimeth-Oxysilane' Self-Assembled Effect
Self-Assembled Monolayers
Recent worldwide research on self-assembled monolayers (SAMs) on silicon substrates has witnessed a tremendous potential through demonstrations of various molecular electronic devices, such as, rectifiers, memories, resonant tunnel diodes etc. The deposition of high-quality 3-aminopropyltrimethoxysilane monolayers have been reported by several groups. Characterization of the multilayers using static de-ionized water contact angle, ellipsometry, X-ray photoelectron spectroscopy and atomic force microscope measurements revealed that self-assembling of the multilayers takes place in two distinct stages: (i) the first 3-aminopropyltrimethoxysilane monolayer chemisorbs on a hydroxylated oxide surface by a silanization process and, (ii) the surface amino group of the first monolayer chemisorbs the hydrolyzed silane group of other 3-aminopropyltrimethoxysilane molecules present in the solution, leading to the formation of a bilayer. The second stage is a self-replicating process that results in the layer-bylayer self-assembly of the multilayers with trapped NH3+ ions. The current–voltage characteristics of the multilayers exhibit a hysteresis effect along with a negative differential resistance, suggesting their potential application in the molecular memory devices. [1]
Structural Features of Self-Assembly
In this work freshly prepared self-assembled 3-aminopropyltrimethoxysilane films on silicon oxide were investigated using XPS, SIMS and AFM techniques. XPS measurements indicated the complete coating of the silicon wafer and confirmed successful deposition. Furthermore, XPS maps showed a uniform elemental distribution on the macroscopic scale, indicating that a continuous and homogeneous 3-aminopropyltrimethoxysilane film covered the whole silicon surface. SIMS was used for the acquisition of wide spectra, depth profiles, negative and positive ion maps. Depth profiles indicated a high molecular order for the 3-aminopropyltrimethoxysilane chains and suggested the presence of a thin water layer adsorbed on top of the self-assembled film. An approximate 3-aminopropyltrimethoxysilane thickness estimate was given by SRIM calculations. Both negative and positive ion detection modes indicated that the 3-aminopropyltrimethoxysilane film was most likely formed by a maximum of two layers. All the SIMS maps showed a highly homogeneous brightness distribution on a micrometer scale and confirmed the presence of an adsorbed water layer on top of the surface.0 10 nm. Surface morphology was studied by tapping mode and phase imaging AFM on a nanometre scale. All the pictures showed high surface homogeneity, and the absence of domain structures was proof of the absence of vertical 3-aminopropyltrimeth-oxysilane polymerization. [2]

Enhancement of The Performance of Metallic Materials
Magnesium based alloys are attracting tremendous interests as the novel biodegradable metallic biomaterials. However, the rapid in vivo degradation and the limited surface biocompatibility restrict their clinical applications. In the present study, in order to improve the corrosion resistance and surface biocompatibility, magnesium alloy (AZ31B) was modified by the alkali heating treatment followed by the self-assembly of 3-phosphonopropionic acid, 3-aminopropyltrimethoxysilane (APTMS) and dopamine, respectively. The results indicated that the molecules were successfully immobilized on the magnesium alloy surface by the self-assembly. An excellent hydrophilic surface was obtained after the alkali heating treatment and the water contact angle increased to some degree after the self-assembly of dopamine, 3-aminopropyltrimethoxysilane and 3-phosphonopropionic acid. [3]
The use of nano-scale particles in the polymeric coatings has become an efficient method to improve their mechanical, optical, physical, anticorrosion, electrical and thermal properties. Fe2O3 nanoparticles were modified with various amounts of 3-aminopropyltrimethoxysilane. Modified and unmodified nanoparticles were introduced into the polyurethane matrix at different concentrations. The FTIR spectra and XPS analysis clearly showed that 3-aminopropyltrimethoxysilane was grafted on the surface of nanoparticles successfully and formed chemical bonds with the surface. Also, surface treatment of the nanoparticles by silane resulted in the significant improvement of the mechanical properties of the polyurethane coating. [4]
Applications of Food Packaging Films and Coatings
An antimicrobial pullulan derivative was prepared by chemical grafting of aminopropyl groups using a silane coupling agent (3-aminopropyltrimethoxysilane). The functionalization of pullulan was confirmed by infrared and nuclear magnetic resonance spectroscopy, and the ensuing transparent films were characterized in terms of thermal stability, mechanical properties and antimicrobial activity toward Staphylococcus aureus and Escherichia coli. The functionalized pullulan showed antimicrobial activity toward S. aureus and E. coli attributed to the presence of aminopropyl groups in the pullulan chains. These materials offer a promising strategy to develop new films and coatings for dry food packaging as well as for biomedical applications because of the combination of the supplementary antimicrobial properties with the thermal and mechanical properties associated to pullulan materials. [5]
![]()
![]() References:
References:
[1] Chauhan, A., Aswal, D., Koiry, S. et al. Self-assembly of the 3-aminopropyltrimethoxysilane multilayers on Si and hysteretic current–voltage characteristics. Appl. Phys. A 90, 581–589 (2008). http://doi.org/10.1007/s00339-007-4336-7
[2] Allen, G. C., Sorbello, F., Altavilla, C., Castorina, A., & Ciliberto, E. (2005). Macro-, micro-and nano-investigations on 3-aminopropyltrimethoxysilane self-assembly-monolayers. Thin Solid Films, 483(1-2), 306-311.
[3] Pan, C. J., Hou, Y., Wang, Y. N., Gao, F., Liu, T., Hou, Y. H., ... & Wang, L. R. (2016). Effects of self-assembly of 3-phosphonopropionic acid, 3-aminopropyltrimethoxysilane and dopamine on the corrosion behaviors and biocompatibility of a magnesium alloy. Materials Science and Engineering: C, 67, 132-143.
[4] Palimi, M. J., Rostami, M., Mahdavian, M., & Ramezanzadeh, B. (2014). Surface modification of Fe2O3 nanoparticles with 3-aminopropyltrimethoxysilane (APTMS): An attempt to investigate surface treatment on surface chemistry and mechanical properties of polyurethane/Fe2O3 nanocomposites. Applied surface science, 320, 60-72.
[5] Fernandes, S. C., Sadocco, P., Causio, J., Silvestre, A. J., Mondragon, I., & Freire, C. S. (2014). Antimicrobial pullulan derivative prepared by grafting with 3-aminopropyltrimethoxysilane: Characterization and ability to form transparent films. Food Hydrocolloids, 35, 247-252.
- Related articles
- Related Qustion
Sodium taurocholate aids drug crystallization inhibition, is key to NTCP-mediated bile acid transport , and forms micelles for PAH fluorometrix analysis.....
Sep 2,2025Biochemical EngineeringAs a collagen peptide, hexapeptide-9 has been designed to provide curative and/or preventive treatment for skin aging and to enhance skin appearance.....
Sep 4,2025Amino Acids and Derivatives3-Aminopropyltrimethoxysilane
13822-56-5You may like
3-Aminopropyltrimethoxysilane manufacturers
- 3-Aminopropyltrimethoxysilane
-

- $10.00 / 1KG
- 2025-10-17
- CAS:13822-56-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- 3-Aminopropyltrimethoxysilane
-

- $0.00 / 1kg
- 2025-09-30
- CAS:13822-56-5
- Min. Order: 0.0001kg
- Purity: 99%
- Supply Ability: 2000000T
- 3-Aminopropyltrimethoxysilane
-
- $100.00 / 1KG
- 2025-09-25
- CAS:13822-56-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available






