Ethyl S-4-Chloro-3-Hydroxybutyrate: Bioreduction Synthesis & Production Enhancement
Oct 27,2025
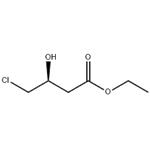
Ethyl S-4-chloro-3-hydroxybutyrate is a chiral intermediate generally used to prepare atorvastatin, a cholesterol-lowering drug. It can be used as a starting material in the synthesis of 4-amino-3-hydroxybutyric acid, a compound of pharmacological importance.

Highly efficient synthesis of ethyl S-4-chloro-3-hydroxybutyrate and its derivatives
Ethyl S-4-chloro-3-hydroxybutyrate [(S)-CHBE] is a key intermediate for the production of chiral drugs, including cholesterol-lowering HMG-CoA reductase inhibitors such as Lipitor. Therefore, a more practical way to synthesize highly optical active of (S)-CHBE (>99.9% ee) is of great interest. Compared with conventional chemical synthesis, the asymmetric bioreduction of ethyl 4-chloro-3-oxobutanoate (COBE), which is inexpensive and easily synthesized, is an economical approach to the production of (S)-CHBE. However, the asymmetric biotransformation of COBE to Ethyl S-4-chloro-3-hydroxybutyrate by reductases often require cofactor NADH or NADPH as an electron donor. Because of the high cost of these cofactors, in situ cofactor regeneration is an effective approach to the economic viability of industrial-scale biotransformations
. Enzyme-coupled approach and substrate-coupled system could be employed as the efficient and cost-effective cofactor recycling systems. For example, glucose dehydrogenase could be used as enzyme-coupled system for recycling NAD+ or NADP+ ; isopropanol could be chosen as cosubstrate for improving the yield of Ethyl S-4-chloro-3-hydroxybutyrate. Although some co-expression systems have been used for designing these recycling systems, few high-level co-expression of the enzymes against 3000 mM COBE were obtained with high enzyme activity and excellent enantioselectivity in the monophasic aqueous media. To asymmetrically synthesize (S)-CHBE in the highly efficient process, it is necessary to screen for the appropriate reductases.[1]
To effectively synthesize Ethyl S-4-chloro-3-hydroxybutyrate from 3000 mM COBE in a small scale, a 100 mL reaction mixture of PBS buffer (100 mM, pH 7.0), 49.38 g COBE, 0.45 mol glucose, 10 g wet cells, NAD+ (30 μmol), MnCl2 (10 μmol) in a 500-mL flask was used for the biotransformation. The pH was adjusted to 7.0 with 2 M NaOH. After stirred at 300 rpm for 14 h, the reaction was obtained in the conversion of 100%, and the ee value of the product was excellent (>99.9% ee). The reductase (CmCR) from recombinant E. coli CCZU-K14 displayed high reductase activity and excellent stereoselectivity for the bioreduction of COBE and its derivatives using glucose as cosubstrate. After the reaction optimization, the optimum cosubstrate concentration, NAD+ concentration, reaction temperature, reaction pH, additive, substrate concentration and cell dosage were 1.5 mmol glucose/(mmol COBE), 0.1 μmol NAD+/(mmol COBE), 30 °C, 7.0, Mn2+ (0.1 mM), 0.1 g (wet weight)/mL, and 1000 mM, respectively. Furthermore, high concentration of COBE (3000 mM) could be asymmetrically reduced to Ethyl S-4-chloro-3-hydroxybutyrate with high yield (99.0%) and excellent ee (>99.9%) after 14 h.
Enhancement of Ethyl S-4-chloro-3-hydroxybutyrate production
Optically active ethyl 4-chloro-3-hydroxybutanoate (CHBE) is a key intermediate in the synthesis of pharmacologically active compounds. The (R)-enantiomer is a useful chiral building block for l-carnitine, whereas the (S)-enantiomer is a precursor of hydroxymethylglutaryl-CoA reductase inhibitors such as Lipitor, which is the top-selling pharmaceutical product in the world. However, the biocatalysis of COBE to (S)- or (R)-CHBE by reductases often requires the presence of the expensive cofactor NADH or NADPH to donate electrons. Effective means for cofactor regeneration, such as through a substrate- and enzyme-coupled system, have attracted attention to reduce the high cofactor costs. Usually, 2-propanol is chosen as a cosubstrate for producing Ethyl S-4-chloro-3-hydroxybutyrate. Several reductases have already been cloned and used for the asymmetric synthesis of (S)-CHBE, such as SOU1 from Candida albicans, S1 from Candida magnoliae, and the carbonyl reductase ScCR from Streptomyces coelicolor . However, the inhibition of the reaction by the substrate or product is still a key limitation to the industrial application of these enzymes. Many attempts have been made to solve this problem. A water/toluene biphasic system was employed for the asymmetric reduction of COBE to Ethyl S-4-chloro-3-hydroxybutyrate to avoid substrate and product inhibition effects.[2]
In summary, a carbonyl reductase (SrCR) from Synechocystis sp. PCC 6803 was cloned, overexpressed in E. coli BL21(DE3), and purified to homogeneity. Furthermore, the recombinant E. coli strain coexpressing SrCR and BsGDH showed high catalytic activity in (S)-CHBE production, with excellent enantioselectivity (99.4% ee). An in situ adsorption system wasd successfully used to overcome the inhibition by Ethyl S-4-chloro-3-hydroxybutyrate, resulting in significant increase of the substrate concentration to 3000 mM (494 g/L), enhancing the overall efficiency and productivity.
References
[1]He, Y. -C., Tao, Z. -C., Zhang, X., Yang, Z. -X., & Xu, J. -H. (2014). Highly efficient synthesis of ethyl (S) -4 -chloro -3 -hydroxybutanoate and its derivatives by a robust NADH -dependent reductase from E. coli CCZU -K14. Bioresource Technology, 161, 461–464. http://doi.org/10.1016/j.biortech.2014.03.133
[2]Chen, L. -F., Fan, H. -Y., Zhang, Y. -P., Wei, W., Lin, J. -P., Wei, D. -Z., & Wang, H. -L. (2017). Enhancement of ethyl (S) -4 -chloro -3 -hydroxybutanoate production at high substrate concentration by in situ resin adsorption. Journal of Biotechnology, 251, 68–75. http://doi.org/10.1016/j.jbiotec.2017.04.014
- Related articles
- Related Qustion
Corosolic acid boosts insulin sensitivity, modulates cancer pathways (NF-κB/PI3K/Akt), and has analogs with improved bioavailability.....
Oct 27,2025APIIsobutaneboronic acid uses DAN-derivatization to avoid GC column damage, enabling accurate residual solvent detection per ICH guidelines.....
Oct 27,2025APIEthyl S-4-chloro-3-hydroxybutyrate
86728-85-0You may like
Ethyl S-4-chloro-3-hydroxybutyrate manufacturers
- Ethyl S-4-chloro-3-hydroxybutyrate
-
- 2025-10-27
- CAS:86728-85-0
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Afatinib impurity 38
-

- $0.00 / 10mg
- 2025-09-25
- CAS:86728-85-0
- Min. Order: 10mg
- Purity: 98%
- Supply Ability: 500mg
- Ethyl S-4-chloro-3-hydroxybutyrate
-

- $8.00 / 1KG
- 2025-09-25
- CAS:86728-85-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available






