Structural Characteristics and Synthesis of Canthaxanthin
Oct 22,2025
Canthaxanthin (INS161g) is primarily used as a feed additive for animals. It can lead to a more intensely-coloured egg yolk and flesh from poultry, and a more reddish colour for salmon. It can also be used as a colour additive in foods. Nevertheless, its use for such purpose is less common than as feeding stuffs for animals. High value ketocarotenoids, such as astaxanthin and canthaxanthin, are highly appealing for applications in human nutraceutical, cosmetic, and animal feed industries due to their color- and health-related properties.

Figure 1: Picture of Canthaxanthin
General Introduction
Canthaxanthin is a keto-carotenoid pigment widely distributed in nature, carotenoids belong to a larger class of phytochemicals known as terpenoids. Canthaxanthin was first isolated in edible mushrooms, It has also been found in green algae, bacteria, crustaceans, and bioaccumulates in fish such as carp, golden grey mullet, seabream and trush wrasse. Canthaxanthin is a type of carotenoid pigment with an orange-red colour. Canthaxanthin occurs naturally in many foods such as mushrooms, crustaceans, fish and eggs. The pigment has also been produced by chemical synthesis. [1]
Structural Characteristics
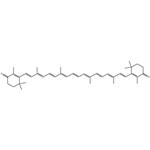
In nature, the thermodynamically more stable all-trans-canthaxanthin isomer is mainly observed in carotenoid extracts. Cis-double bonds, present in the cis-canthaxanthins, create greater steric hindrance between nearby hydrogen atoms or methyl groups, resulting in their lower stability. Nonetheless, conditions of temperature, acidity, or light can promote isomerization of the trans- to the cis-forms, resulting in 9-cis- and 13-cis-canthaxanthin and 13 more cis-isomers that have been identified. The effect of isomerization has been investigated, in particular the various apoptotic and antioxidant activities of each isomer. [1]
Synthesis

Figure 2: Synthetic route of Canthaxanthin
Biosynthesis
Due to the commercial value of Canthaxanthin, their biosynthesis has been studied extensively in both natural producers, and non-natural (heterologous) systems such as the bacteria Escherichia coli and yeast Saccharomyces cerevisiae. Canthaxanthin biosynthesis proceeds from beta-carotene via the action of a single protein, known as a beta-carotene ketolase, that is able to add a carbonyl group to carbon 4 and 4' of the beta carotene molecule. Although functionally identical, several distinct beta-carotene ketolase proteins are known.
Chemical synthesis
The first synthesis of canthaxanthin is shown in figure 1, 6-oxo-isophorone was converted after several steps into the corresponding C15-Wittig salt 8, which is a key intermediate for the canthaxanthin synthesis. Z/E-canthaxanthin was obtained via a double Wittig olefination by mixing two equivalents of compound 8 and the symmetrical building block C10-dialdehyde 9. The two canthaxanthin isomers can be separated by crystallization, since the all-E-canthaxanthin is poorly soluble. The main drawback of this Wittig olefination is the formation of triphenylphosphine oxide as a by-product, which makes the reaction atom-inefficient, and it is also di cult to separate from the desired reaction product. [1]
Safety of Canthaxanthin
The carotenoid pigment ‘canthaxanthin’ has been used in aquafeed for many years in order to impart the desired flesh colour in farmed salmonids. A side effect of extreme over dosage of this carotenoid is the deposition of minute crys tals in the eye, a fact leading to adverse media attention for canthaxanthin in the past, and some pressure to limit its use aquafeeds. A real appraisal of the possible amount of can thaxanthin ingested by humans through the consumption of notable quantities of highly pigmented salmon, reveals that it is extremely unlikely that consumers could be exposed to sufficient canthaxanthin to attain the estab lished ADI for this pigment. Additionally, the ADI is 10 times lower than the reported no-effect level (NOEL) for canthax anthin. Therefore, canthaxanthin is a safe source of car otenoids for salmonid feeds. [2]
Application field
In food and feed additives, canthaxanthin is used to enhance the color of eggs and aquatic products, making the egg yolksmore ruddy and the fish meat more vibrant. In the pharmaceutical field, canthaxanthin exhibits biological activities such asantioxidant, immune modulation, and anti-inflammatory properties. Studies have found that it possesses certaintherapeutic potential for conditions like atopic dermatitis. In addition, due to its antioxidant properties, it can be used inskin care products to help protect against free radical damage.
References
[1] Bárbara A. Rebelo, Plants 2020, 9, 1039.
[2] Rémi T.M Baker, Trends in Food Science & Technology, 2001, 12: 240-243.
- Related articles
- Related Qustion
Canthaxanthin
514-78-3You may like
- canthaxanthin
-

- $0.00 / 1kg
- 2025-10-22
- CAS:514-78-3
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 2000kg
- Canthaxanthin
-

- $0.00 / 1kg
- 2025-10-22
- CAS:514-78-3
- Min. Order: 1kg
- Purity: 99.3%
- Supply Ability: 10mt
- Canthaxanthin
-
- 2025-10-22
- CAS:514-78-3
- Min. Order:
- Purity: 0.99
- Supply Ability:




