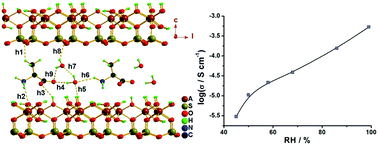
In this study, an intercalated hybrid solid of kaolinite with L-alanine (abbr. K-Ala) was prepared, and the proton conductance of K-Ala has been investigated in both anhydrous and humid environments, respectively. The proton conductivity (σ) of K-Ala is 5.38 × 10−8 S cm−1 under an N2 atmosphere (anhydrous environment), while it is much enhanced under humid conditions and σ = 1.35 × 10−4 S cm−1 under 99% relative humidity (RH) at ambient temperature. With increasing temperature, the proton conductivity reaches 2.1 × 10−3 S cm−1 at 99% RH and 318 K, and this σ value is comparable to that recently reported in some of the high proton conducting MOF/PCP materials. DFT calculations were performed for the crystal structures containing different amounts of water molecules within the interlayer spaces of K-Ala, disclosing that the amounts of water molecules strongly influence the H-bond network. A denser H-bond network is formed when there are large amounts of water molecules in the interlayer spaces of K-Ala, providing an efficient proton transport pathway; as a consequence, the proton conductivity of K-Ala is much enhanced at high relative humidity.