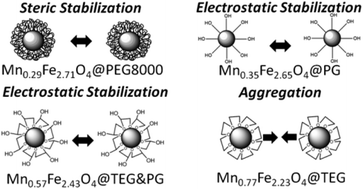
Manganese doped ferrite (MnxFe3−xO4) nanoparticles with x = 0.29–0.77 were prepared under solvothermal conditions in the presence solely of a polyol using the trivalent manganese and iron acetylacetonates as precursors. In this facile approach, a variety of polyols such as polyethylene glycol (PEG 8000), tetraethylene glycol (TEG), propylene glycol (PG) and a mixture of TEG and PG (1 : 1) were utilized in a triple role as a solvent, a reducing agent and a surface-functionalizing agent. The composition of the fine cubic-spinel structures was found to be related to the reductive ability of each polyol, while determination of structural characteristics plus the inversion parameter (i = 0.18–0.38) were provided by X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) spectroscopy at both the Fe and Mn K-edges. The saturation magnetization increased up to 80 emu g−1 when x = 0.35 and i = 0.22. In addition, the as-prepared nanocrystals coated with PEG, PG and PG&TEG showed excellent colloidal stability in water, while the TEG-coated particles were not water dispersible and converted to hydrophilic when were extra PEGylated. Measurements of the 1H NMR relaxation in water were carried out and the nanoprobes were evaluated as potential contrast agents.