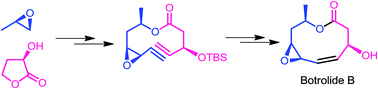
An efficient total synthesis of botryolide B was achieved in 9 longest linear steps with 22% overall yield via esterification of a carboxylic acid with an alcohol fragment and a ring closing metathesis (RCM) reaction as pivotal steps to construct the macrolactone ring system. Our novel approach for the synthesis of the 2-alkene-1,5-diol fragment was achieved by a ring closing metathesis reaction followed by a reductive opening strategy, whereas the carboxylic acid fragment was accessed from commercially available (R)-(+)-α-hydroxy-γ-butyrolactone in three steps.