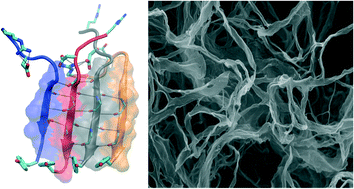
The properties of self-assembling peptides can be tuned through changes at their sequence level, and thus amyloid-forming peptides are increasingly gaining interest as potential biomaterials for a series of applications. Recently, we rationally designed a self-assembling peptide with sequence RGDSGAITIGC, which comprises the amyloidogenic core GAITIG, extracted from the adenovirus fiber shaft, as well as the functional motif RGD and a cysteine residue encompassing cell attachment properties and an exposed free thiol group, respectively. Here, we aimed to further stabilize the aforementioned amyloid fibril by modifying the last residue position and observed that aromatic residues, including tyrosine, contribute to the stabilization of the amyloid fibril through the formation of π–π interactions in the parallel β-sheets formed by the peptides. Inspired by the proximity of tyrosine–tyrosine residues in the intermolecular aromatic network of the newly designed fibrils, we computationally investigated the capacity of a designed amyloid-forming peptide to concurrently possess dityrosine crosslinking formation potential in addition to cell adhesive properties. We experimentally confirmed the ability of the designer peptides to self-assemble into amyloid-type fibrils. The designed amyloid peptide biomaterials may constitute promising agents in future tissue engineering applications.