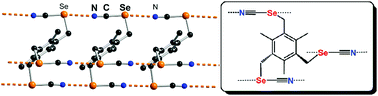
Intermolecular chalcogen bonding interactions are identified in crystalline organic selenocyanates where a linear Se⋯N![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) C interaction takes place, leading to the recurrent formation of chain-like motifs ⋯Se(R)–CN⋯Se(R)–CN⋯, stabilized by cooperativity. Analysis of 15 reported structures of such selenocyanates is complemented by the structural determinations of three other novel polytopic selenocyanates, namely 1,3,5-tris(selenocyanatomethyl)benzene (1a), 1,3,5-tris(selenocyanatomethyl)-2,4,6-trimethylbenzene (1b) and 1,2,4,5-tetrakis(selenocyanatomethyl)benzene (2). While the recurrent chain-like motifs with short and linear Se⋯N contacts are indeed observed in the pure compounds, solvates with DMF and AcOEt also demonstrate that the nitrile N atom can be easily displaced from the chalcogen bond by stronger Lewis bases such as carbonyl oxygen atoms, leading in the case of (2)·(DMF)2 to a chelating motif where two neighboring CH2–SeCN groups link to the same oxygen atom through Se⋯O interactions.
C interaction takes place, leading to the recurrent formation of chain-like motifs ⋯Se(R)–CN⋯Se(R)–CN⋯, stabilized by cooperativity. Analysis of 15 reported structures of such selenocyanates is complemented by the structural determinations of three other novel polytopic selenocyanates, namely 1,3,5-tris(selenocyanatomethyl)benzene (1a), 1,3,5-tris(selenocyanatomethyl)-2,4,6-trimethylbenzene (1b) and 1,2,4,5-tetrakis(selenocyanatomethyl)benzene (2). While the recurrent chain-like motifs with short and linear Se⋯N contacts are indeed observed in the pure compounds, solvates with DMF and AcOEt also demonstrate that the nitrile N atom can be easily displaced from the chalcogen bond by stronger Lewis bases such as carbonyl oxygen atoms, leading in the case of (2)·(DMF)2 to a chelating motif where two neighboring CH2–SeCN groups link to the same oxygen atom through Se⋯O interactions.