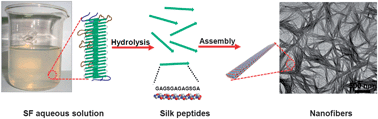
To obtain the peptides with various functions, the “bottom-up” methods including chem- or bio-synthesis are historically emphasized, in which the sequences of peptide are designed on purpose. In this paper, we present the reverse way, i.e. a “top-down” approach, to harvest the peptides by enzyme hydrolysis of Bombyx mori silkworm silk fibroin (SF), a kind of natural fibrous protein with relatively simple motifs. It is found that the elastase attacks the peptide bond between alanine (A) and glycine (G) in the edges of SF fibril (turning points of the SF chain which folds cross β-sheet) prior to the one in the regular (crystalline) region during the enzymolysis. Therefore, a dodecapeptide GAGSGAGAGSGA in the final product with a relatively high yield confirmed the cross β-sheet structure model for the SF fibrils we proposed in previous work. Moreover, the obtained dodecapeptide is able to form β-sheet, and further assemble into nanofibers with a temperature-responsive behavior. Based on these results, a model is proposed to interpret the structure of the self-assembled dodecapeptide.