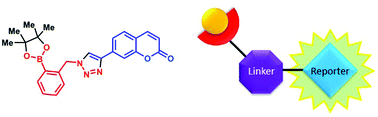
A series of boronic acid-containing saccharide receptors was synthesised via copper catalysed azide–alkyne cycloaddition (CuAAC) reactions. Their saccharide binding capacity was studied by 1H and 11B NMR spectroscopy titrations and isothermal titration calorimetry (ITC) techniques. Fluorescent sensors were generated by linking a phenylboronic acid (PBA) receptor with fluorophores via a triazole-linker. Fluorescence titrations with fructose revealed that the substitution pattern about the PBA influences the fluorescence response to saccharides. Titrations studied by 1H NMR spectroscopy suggested that fructose binding is enhanced when the aromatic ring bearing the boronic acid has the triazole-containing substituent at the ortho position. No evidence of either a dative N–B bond or solvent insertion (between B and N) was observed by 11B NMR spectroscopy. These results demonstrate that synthetic accessible triazole receptors may allow rapid sensor synthesis, screening and discovery.