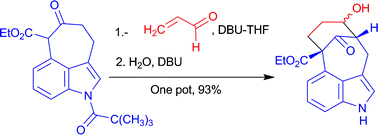
An efficient synthesis of the N-methylwelwistatin tetracyclic core in only two steps from Kornfeld's ketone is described, whose key transformation involves the generation of a fused bicyclo[4.3.1]decane ring system through a one-pot sequence comprising a Michael-intramolecular aldolization anionic domino process and a DBU-promoted hydrolysis of the N-pivaloyl protecting group. Besides providing the most efficient synthesis of the welwistatin core to date, this method has the advantage of installing an oxygenated function at the welwistatin D ring.