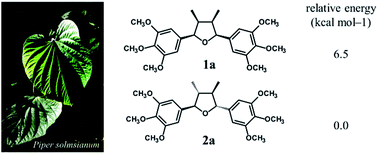
The study of variability of the tetrahydrofuran lignan (−)-grandisin in leaves of Piper solmsianum (Piperaceae) revealed two unknown compounds, that were isolated and determined to be the all-cis tetrahydrofuran lignans 1a [rel-(7R,8S,7′S,8′R)-3,4,5,3′,4′,5′-hexamethoxy-7,7′-epoxylignan] and 1b [rel-(7R,8S,7′S,8R′)-3′,4′-methylenedioxy-3,4,5,5′-tetramethoxy-7,7′-epoxylignan]. Their structures were determined by spectroscopic analysis including 1D and 2D-NMR while their configurations were determined by ECD associated to the density functional theory (DFT) at the B3LYP/6-31G(d,p) level. The hydrogen bonds between methoxy groups in trimethoxyphenyl rings stabilizes the all-trans tetrahydrofuran lignan grandisin by 6.5 kcal mol−1 as compared to the corresponding all-cis isomer of grandisin. The occurrence of all-cis tetrahydrofuran lignans as natural products is a very rare event.