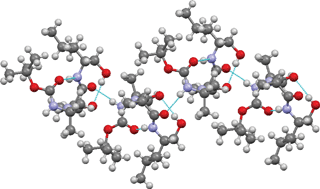
A facile, efficient and racemization-free method for the synthesis of N-protected β-amino alcohols and peptaibols using N-hydroxysuccinimide active esters is described. Using this method, dipeptide, tripeptide and pentapeptide alcohols were isolated in high yields. The conformations in crystals of β-amino alcohol, dipeptide and tripeptide alcohols were analysed, with a well-defined type III β-turn being observed in the tripeptide alcohol crystals. This method is found to be compatible with Fmoc-, Boc- and other side-chain protecting groups.