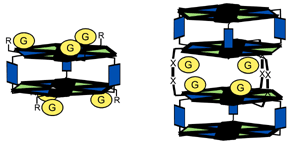
This article describes the complexation of phenol derivatives by hydrogen-bonded receptors. These phenol receptors are formed by self-assembly of calix[4]arene dimelamine or tetramelamine derivatives with 5,5-diethylbarbiturate (DEB) or cyanurate derivatives (CYA). The double rosette assemblies 33·(DEB)6/(CYA)6 have their phenol-binding functionalities (ureido groups) at the top and at the bottom of the double rosette (exo-receptors). The tetrarosette assemblies 43·(DEB)12/(CYA)12 form a cavity with binding sites between the two double rosettes for guest encapsulation (endo-receptors). An intrinsic binding constant Ka of 202 M−1 and 286 M−1 for the binding of 4-nitrophenol to the ureido functionalized exo- and endo-receptors, respectively, was observed. For the exo-receptor a 1 : 6 stoichiometry was observed while for the endo-receptor 1 : 4 binding stoichiometry was determined by Job plot and MALDI-TOF MS. The important role that the hydroxy group's acidity plays in the complexation of 4-nitrophenol is clarified by binding studies with different phenol derivatives. The hydrogen-bonded receptors showed a much smaller response towards less acidic phenol derivatives.