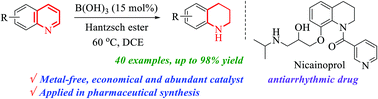
Boric acid promoted transfer hydrogenation of substituted quinolines to synthetically versatile 1,2,3,4-tetrahydroquinolines (1,2,3,4-THQs) was described under mild reaction conditions using a Hantzsch ester as a mild organic hydrogen source. This methodology is practical and efficient, where isolated yields are excellent and reducible functional groups are well tolerated in the N-heteroarene moiety. The reaction parameters and tentative mechanistic pathways are demonstrated by various control experiments and NMR studies. The present work can also be scaled up to obtain gram quantities and the utility of the developed process is illustrated by the transformation of 1,2,3,4-THQs into a series of biologically important molecules including the antiarrhythmic drug nicainoprol.