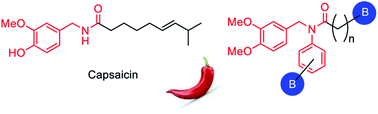
This study reports on the preparation of eight new boron-containing capsaicinoids bearing long aliphatic chains, as an expansion of our previous studies to include tertiary amide derivatives into our substrate scope. Our boron-moiety, a pinacolboronate ester (Bpin) fragment, has been incorporated in two locations: as an aryl substituent of the capsaicinoid produced by the reductive amination of veratraldehyde, or at the terminal end of an aliphatic substituent using an iridium catalyzed hydroboration reaction. We report that most compounds in our series show moderate antimicrobial and cytotoxic activity, surpassing activities noted in our previous study.