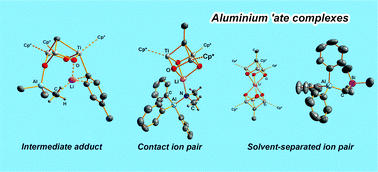
Treatment of [{Ti(η5-C5Me5)(μ-O)}3(μ3-CR)] [R = H (1), Me (2)] with AlPh3 or AlMe3 gives adducts [R′3Al(μ3-O)(μ-O)2{Ti(η5-C5Me5)}3(μ3-CR)] [R = H, R′ = Ph (3), Me (4); R = Me, R′ = Ph (5), Me (6)] that react with LiNMe2 to give the contact ion pair complexes [Ph3Al(μ-NMe2)Li(μ3-O)3{Ti(η5-C5Me5)}3(μ3-CR)] [R = H (7), Me (8)] or the solvent-separated ion pair compounds [Li{(μ3-O)3Ti3(η5-C5Me5)3(μ3-CR)}2][(Me3Al)2(μ-NMe2)] [R = H (9), Me (10)]. Reactions of 1 or 2 with a mixture of AlPh3 and LiCH2SiMe3 lead to the solvent-separated ion pair complexes [Li{(μ3-O)3Ti3(η5-C5Me5)3(μ3-CR)}2][Al(CH2SiMe3)Ph3] [R = H (11), Me (12)], presumably by evolution of redistribution intermediates containing the lithium dicubane cation [Li{(μ3-O)3Ti3(η5-C5Me5)3(μ3-CR)}2]+ and [Li{Al(μ-Ph)2Ph(CH2SiMe3)}2]− anionic units. Surprisingly, reaction of 4 with p-tolyl lithium gives the complex [{Me2Al(μ-Me)Li(p-MeC6H4)}{(μ3-O)2(μ-O)Ti3(η5-C5Me5)3(μ3-CH)}] 13 in which the aryl lithium species is incorporated showing interactions with both the trialkyl aluminium unit and the organometallic oxide 1. X-ray diffraction studies were performed on 8, 12 and 13.