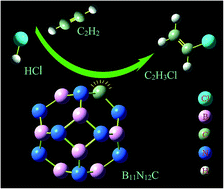
Density functional theory calculations were used to investigate the mechanism of acetylene hydrochlorination separately catalyzed by un-doped B12N12 and carbon-doped BN fullerene (B12−nN11+nC (n = 0, 1)). We have discovered that carbon-doped BN clusters displayed extraordinary catalyst performance for acetylene hydrochlorination compared with un-doped B12N12 clusters. C2H2 was adsorbed onto B12−nN11+nC (n = 0, 1) clusters prior to HCl and then formed three adsorption states. The first two states were in a trans configuration, in which the two H atoms of C2H2 were on opposite sides of the C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C bond; the third state was a cis configuration, in which the two H atoms were on the same side of the C
C bond; the third state was a cis configuration, in which the two H atoms were on the same side of the C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C bond. Afterwards, we illustrated three possible pathways with corresponding transition states. In particular, the minimum energy pathway R1 based on the B11N12C catalyst had an energy barrier as low as 36.08 kcal mol−1, with only one transition state.
C bond. Afterwards, we illustrated three possible pathways with corresponding transition states. In particular, the minimum energy pathway R1 based on the B11N12C catalyst had an energy barrier as low as 36.08 kcal mol−1, with only one transition state.