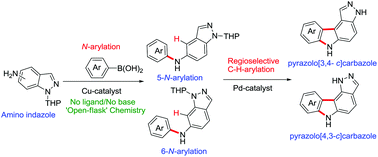
Cu(II)-catalyzed cross-coupling of various aryl boronic acids with 5 and 6-amino indazoles has resulted in (arylamino)-indazoles. These (arylamino)-indazoles have been utilized in synthesizing medicinally important pyrazole-fused carbazoles via Pd(II)-catalyzed cross-dehydrogenative coupling (CDC). This combined N-arylation/C–H arylation strategy has been successfully applied to the regioselective synthesis of polyheterocycles 3,6-dihydropyrazolo[3,4-c]carbazoles and 1,6-dihydro pyrazolo[4,3-c]carbazoles. Quantum chemical analysis has been carried out to understand the regioselectivity and to trace the potential energy surface of the entire reaction upon 5-N-aryl-indazole conversion to the corresponding carbazole.