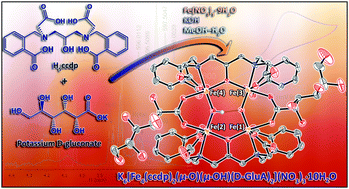
A novel tetra-iron(III) complex, K6[Fe4(ccdp)2(μ-O)(μ-OH)(D-GluA)2](NO3)3·10H2O (K6[1](NO3)3·10H2O) with two gluconato and two N,N′-bis[2-carboxobenzomethyl]-N,N′-bis[carboxomethyl]-1,3-diaminopropan-2-oxy (ccdp5−) ligands has been synthesized and fully characterized using different techniques including single crystal X-ray crystallography. K6[1](NO3)3·10H2O showed stability aqueous solution, in biological samples such as human and fetal bovine serum (HS and FBS) and cell cultures such as phosphate buffer saline and minimum essential medium (PBS and MEM) using UV-Vis spectroscopy at room temperature and 37 °C. The redox behavior of the complex was studied using cyclic and square wave voltammetries in aqueous and DMF solutions. Furthermore, magnetic study using electron paramagnetic resonance spectroscopy and Guoy balance magnetic moment measurements indicated antiferromagnetic coupling among the metal centers of the (μ-O)(μ-OH) bridged tetra-iron(III) anion core of the complex. Electrochemical studies of the K6[1](NO3)3·10H2O in aqueous and DMF solutions using cyclic and square-wave voltammetries revealed the presence of multi-accessible electron metal-based redox events.