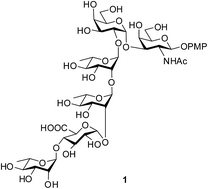
Chemical synthesis of the hexasaccharide, α-L-Rhap-(1→4)-β-D-GlcAp-(1→2)-α-L-Rhap-(1→2)-α-L-Rhap-(1→2)-α-D-Galp-(1→3)-β-D-GalNAcp, is reported by following a [2 + 4] convergent strategy. A disaccharide α-D-Galp-(1→3)-β-D-GalNAcp and a tetrasaccharide α-L-Rhap-(1→4)-β-D-GlcAp-(1→2)-α-L-Rhap-(1→2)-α-L-Rhap were synthesized from commercially available monosaccharides through rational protecting group manipulations and stereoselective glycosylation involving activation of thioglycoside using N-iodosuccinimide and H2SO4-silica. Final glycosylation between the tetrasaccharide donor and the disaccharide acceptor was achieved by the activation of trichloroacetimidate using H2SO4-silica alone. A late stage TEMPO-mediated oxidation installed the required uronic acid moiety. Finally, global deprotection furnished the target molecule. Successful chemical synthesis of the repeating unit of E. coli O120 will help to design suitable vaccine candidates against this deadly pathogen belonging to the STEC family.