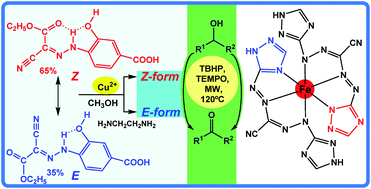
The aquasoluble [Cu(1κN,O2:2κO-HL1)(S)]2 [S = CH3OH (1), (CH3)2NCHO (2)] and [Cu(κN-HL1)(en)2]·CH3OH·H2O (3) CuII complexes were prepared by the reaction of CuII nitrate hydrate with the new ligand (E/Z)-4-(2-(1-cyano-2-ethoxy-2-oxoethylidene)hydrazinyl)-3-hydroxybenzoic acid (H3L1), in the presence (for 3) or absence (for 1 and 2) of ethylenediamine (en), while the FeIII complex [Fe(κN3-HL2)2] (4) was isolated by treatment of iron(III) chloride hexahydrate with the new ligand (1E,1E)-N′,2-di(1H-1,2,4-triazol-3-yl)diazenecarbohydrazonoyl cyanide (H3L2), and characterized by elemental analysis, IR spectroscopy and single crystal X-ray diffraction. Cooperative E,Z → E isomerization of H3L1, induced by coordination and ionic interactions, occurs upon interaction with CuII in the presence of en. Complexes 1–4 act as catalyst precursors for the solvent-free microwave (MW) assisted selective oxidation of primary or secondary alcohols and diols to the corresponding aldehydes, ketones and diketones, respectively, with yields in the 5–99% range (TONs up to 4.96 × 102) after 60 min of MW irradiation at 120 °C. The influence of temperature, time and organic radicals was studied and also the regioselective oxidation of the catalytic systems involving the primary and secondary alcohols.