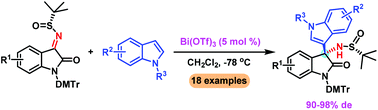
A Lewis acid promoted highly diastereoselective asymmetric Friedel–Crafts alkylation of indoles with isatin-derived N-tert-butanesulfinyl ketimines is described. The reaction can be accomplished with ease in the presence of a catalytic amount of Bi(OTf)3, providing a series of 3-indolyl-3-aminooxindoles in excellent diastereoselectivities (up to 98% de) and yields (up to 99%).