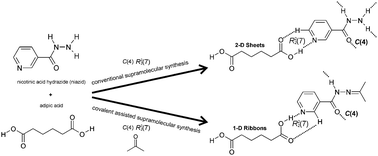
Nicotinic acid hydrazide (niazid) readily co-crystallizes with carboxylic acids in methanol to form a 2-D sheet structure that utilizes all three H bond donors on the carbohydrazide functional group. In acetone solution niazid undergoes a condensation reaction with the solvent, which replaces the amine group with a hydrocarbon group leaving only one hydrogen bond donor on the modified niazid molecule, now containing a N-acylhydrazone functional group. The resulting reduced hydrogen bonding functionality leads to a new supramolecular assembly when co-crystallized with the same dicarboxylic acids.