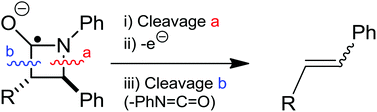
The radical anions of β-lactams, photogenerated in the presence of DABCO as an electron donor, undergo cycloreversion via N–C4 bond cleavage, back electron transfer and final C2–C3 bond cleavage, leading to olefins. The involved intermediates are 1,4-radical anions and 1,4-biradicals. The experimental observations are consistent with the results of DFT calculations.