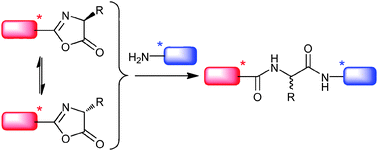
A stereochemical study of a potentially prebiotic peptide-forming reaction was carried out as the first part of a systems chemistry investigation of potential paths for symmetry breaking. Substantial diastereomeric excesses result from a fast epimerization of the 5(4H)-oxazolone intermediate in aqueous solution.