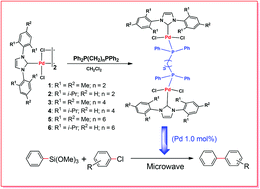
Six dinuclear N-heterocyclic carbene (NHC) palladium complexes, [PdCl2(IMes)]2(μ-dppe) (1), [PdCl2(IPr)]2(μ-dppe) (2), [PdCl2(IMes)]2(μ-dppb) (3), [PdCl2(IPr)]2(μ-dppb) (4), [PdCl2(IMes)]2(μ-dpph) (5), and [PdCl2(IPr)]2(μ-dpph) (6) [IMes = N,N′-bis-(2,4,6-trimethylphenyl)imidazol-2-ylidene; IPr = N,N′-bis-(2,6-di(iso-propyl)phenyl)imidazol-2-ylidene; dppe = 1,2-bis(diphenylphosphino)ethane, dppb = 1,4-bis(diphenylphosphino)butane; and dpph = 1,6-bis(diphenylphosphino)hexane], have been synthesized through bridge-cleavage reactions of chloro-bridged dimeric compounds, [Pd(μ-Cl)(Cl)(NHC)]2, with the corresponding diphosphine ligands. The obtained compounds were fully characterized by 1H NMR, 13C NMR and 31P NMR spectroscopy, FT-IR, elemental analysis and single-crystal X-ray crystallography. Moreover, further explorations of the catalytic potential of the dinuclear carbene palladium complexes as catalysts for the Pd-catalyzed transformations have been performed under microwave irradiation conditions, and the complexes exhibited moderate to good catalytic activity in the Hiyama coupling reaction of trimethoxyphenylsilane with aryl chlorides.