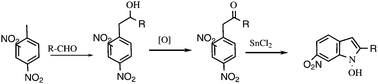
2,4-Dinitrotoluene reacts in the presence of catalytic amounts of DBU with aldehydes to form the corresponding alcohols in good yields (usually above 65%). The obtained alcohols can be readily oxidized to 2,4-dinitrobenzyl ketones, which were used for the synthesis of 6-nitroindoles with various substituents in the 2-position. Starting from non-racemic aldehydes, non-racemic 2-substituted 6-nitroindoles were obtained.