The decolorization of rhodamine B (RhB) using ultrasound (US) combined with a Fenton-like process has been investigated. The effects of operating parameters such as initial dye concentration, pH, and addition of oxalic acid were studied. As expected, the rate and extent of decolorization of RhB increased with rising oxalic acid concentration, but slightly decreased with increasing dye concentration, and exhibited excellent removal efficiency in the wide pH range of 3–9. It was found that, while the degradation rate due to ultrasound alone was slow, the sonication significantly accelerated the Fenton-like reaction. It has been observed that the maximum degradation of 92.16% was obtained under optimum operating conditions of [RhB] = 5 mg L−1; [Oxalic] = 25 mM; pH = 3. The decolorization pattern obeyed first order kinetics and was used to calculate apparent rate constants for dye decolorization. Furthermore, studies in the presence of radical scavengers such as tert-butyl alcohol (TBA), isopropanol (IPA), catalase and L-His on the degradation of RhB were performed, and the results indicated that 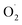 and H2O2 played an important role in this system. Overall it can be said that the combined process resulted in higher extent of degradation as compared to the individual processes based on Fenton type or US irradiation.
and H2O2 played an important role in this system. Overall it can be said that the combined process resulted in higher extent of degradation as compared to the individual processes based on Fenton type or US irradiation.

