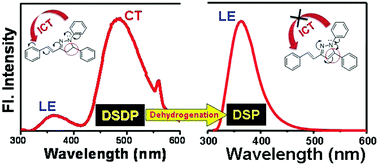
The detailed photophysics of (E)-1,5-diphenyl-3-styryl-4,5-dihydro-1H-pyrazole (DSDP) and (E)-1,5-diphenyl-3-styryl-1H-pyrazole (DSP) has been investigated and compared to demonstrate the drastic modification of the photophysics upon dehydrogenation of the pyrazoline ring. While DSDP gives dual absorption and dual emission bands corresponding to the locally excited (LE) and the intramolecular charge transfer (ICT) species, DSP yields single absorption and emission bands for the locally excited species only. Comparative steady state and time resolved fluorometric studies reveal that aromatization of the pyrazoline ring inhibits the formation of the ICT species. Quantum chemical calculations corroborate and rationalize the inhibition of the ICT process upon aromatization through depiction of the differential electronic distributions in the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of the two fluorophores.