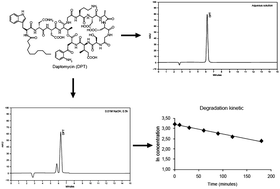
An isocratic liquid chromatography method (LC-UV) was developed and validated to determine daptomycin in injectable form. The method was carried out in a Waters XBridge C18 column (250 mm × 4.6 mm, 5 μm). The mobile phase was composed of methanol–acetonitrile–buffer (pH 2.2) (40 : 30 : 30 v/v/v) at a flow rate of 1.0 mL−1, using photodiode array (PDA) detection at 223 nm. The retention time obtained for daptomycin was 6.1 min and the method was linear in the range of 10 to 50 μg mL−1 (r = 0.9999). Forced degradation studies were performed to verify the specificity and stability-indicating capability of the method. The degradation kinetics under alkaline conditions were also evaluated. The method showed suitable accuracy (99.17%) and precision (RSD 0.59%) A two level full factorial design was used to determine the method robustness. The proposed method was applied for the analysis of daptomycin injectable form, contributing to the improvement of the quality control of this pharmaceutical product.