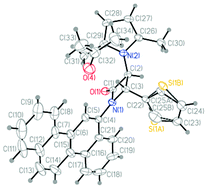
Pyrrole and 2-azetidinone are two essential heterocyclic scaffolds, which are being broadly used in medicinal chemistry and drug discovery field. A green and practical method to synthesize novel N-(2-azetidinonyl) 2,5-disubstituted pyrroles, which are comprised of both pyrrole and 2-azetidinone moieties, has been developed. The classical Paal–Knorr reaction is one of the simplest and most economical methods for the synthesis of biologically important and pharmacologically useful pyrrole derivatives. The present procedure for the synthesis of N-(2-azetidinonyl) 2,5-disubstituted pyrroles (1,2,5-trisubstituted pyrroles) has been accomplished by reacting 3-amino β-lactams and 2,5-hexanedione in the presence of bismuth nitrate pentahydrate as an ecofriendly catalyst using microwave irradiation under solvent free condition. A comparison has also been made between the efficiency of microwave-assisted condition and the classical method (stirring at room temperature). The present method is equally effective for N-polyaromatic substituted β-lactams and optically pure β-lactams with chemically sensitive functionalities. The structure and stereochemistry of the products have been confirmed by extensive spectroscopic and X-ray crystallographic analyses. The present procedure for synthesizing N-(2-azetidinonyl) 2,5-disubstituted pyrroles may find application in the development of potent pharmacologically active molecules.