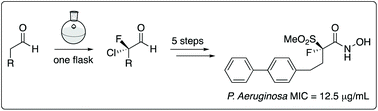
Enantiopure compounds with a strategically incorporated fluorine atom intended to enhance LpxC inhibition have been synthesized using an organocascade fluorination reaction as the key step. These are the first low molecular weight LpxC inhibitors to contain a fluorine atom on a critically important chiral center that is substituted with two pharmacophoric moieties, and were thusly designed to provide new SAR data for this class of compounds. Fluorinated compounds were evaluated against ESKAPE pathogens and exhibited MICs of ≤12.5 μg mL−1 against Pseudomonas aeruginosa.