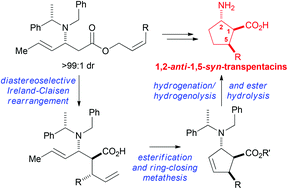
The diastereoselective Ireland–Claisen rearrangement of a range of substituted allyl β-amino esters gave the corresponding enantiopure α-substituted-β-amino esters with good diastereoselectivity. The application of this methodology in the asymmetric synthesis of a range of C(5)-substituted 1,2-anti-1,5-syn-transpentacins was demonstrated by the rearrangement of a range of β-amino esters derived from sorbic acid, followed by esterification, ring-closing metathesis, hydrogenolytic deprotection/reduction, and hydrolysis, which gave the C(5)-substituted transpentacins in only 9 steps from commercially available starting materials.