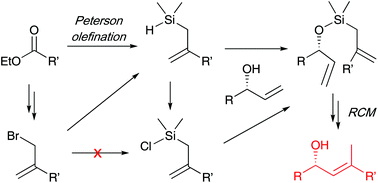
The diastereoselective synthesis of trisubstituted olefins with concomitant C–C bond formation is still a difficult challenge, and olefin metathesis reactions for the formation of such alkenes are usually not high yielding or/and diastereoselective. Herein we report an efficient and diastereoselective synthesis of trisubstituted olefins flanked by an allylic alcohol, by a silicon-tether ring-closing metathesis strategy. Both E- and Z-trisubstituted alkenes were synthesised, depending on the method employed to cleave the silicon tether. Furthermore, this methodology features a novel Peterson olefination for the synthesis of allyldimethylsilanes. These versatile intermediates were also converted into the corresponding allylchlorodimethylsilanes, which are not easily accessible in high yields by other methods.