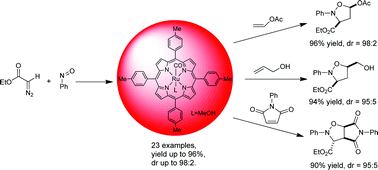
Ruthenium porphyrin catalyzes tandem nitrone formation/1,3-dipolar cycloaddition of diazo compounds, nitrosoarenes and alkenes to form isoxazolidines in good to high yields and with excellent regio-, chemo- and diastereo-selectivities. A broad substrate scope of alkenes is applicable to this protocol and various functional groups are compatible with the reaction conditions. In silico analysis and in vitro biological experiments revealed that some of the new isoxazolidines synthesized in this work could act as leukotriene A4 hydrolase inhibitors.