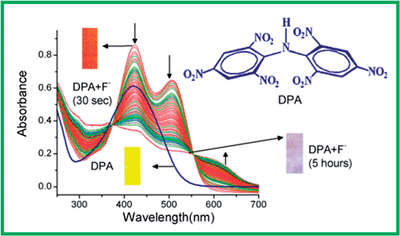
Dipicrylamine (2,4,6-2′,4′,6′-hexanitrodiphenylamine, DPA) has been used for the detection and extraction of metal ions, especially potassium; however, its capability as an anion sensor has not been reported to date. It contains a secondary amine (N–H), the proton of which can form H-bonds with anions. This property has been exploited to investigate the capability of DPA as an anion sensor. Out of the large number of anions used in this study, F−, OAc−, and H2PO4− exhibited strong interactions with sharp colour changes in acetonitrile. The DPA-anion recognition event was monitored by UV-vis, NMR and ESMS studies, apart from distinct colour changes detectable by the bare eye. A detailed investigation revealed that the anions first interact with the N–H proton through H-bonding and then deprotonation takes place forming a DPA−–TBA+ (tetrabutylammonium) complex. The rate constants of these complex formation have been determined from time dependent UV-vis spectral change and the order of the observed rate constants is F− > OAc− > H2PO4−. For F−, the NMR and ESMS data indicated the interaction of F− with one of the carbons or its attached proton in one of the benzene rings. A computational study suggests that the F− ion binds with one of the phenyl carbons instead of the –CH hydrogen bond of DPA−.