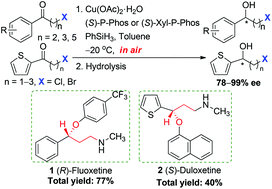
A set of reaction conditions has been established to facilitate the non-precious copper-catalyzed enantioselective hydrosilylation of a number of structurally diverse β-, γ- or ε-halo-substituted alkyl aryl ketones and α-, β- or γ-halo-substituted alkyl heteroaryl ketones under air to afford a broad spectrum of halo alcohols in high yields and good to excellent enantioselectivities (up to 99% ee). The developed procedure has been successfully applied to the asymmetric synthesis of antidepressant drugs (R)-fluoxetine and (S)-duloxetine, which highlighted its synthetic utility.