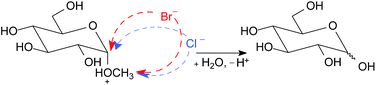
The mechanism of acid hydrolysis of glycoside has been investigated since the end of the 19th century accompanied by lots of literatures published on the mechanism, although little attention has surprisingly been paid to the action of counter anion of acid. In this paper, it was investigated whether or not counter anion of acid directly participates in acid hydrolysis of glycosides, methyl α- and β-D-glucopyranosides (MGP) in water, aqueous 74%, and 82% 1,4-dioxane systems. Because proton activity of a reaction system is the important rate-determining parameter in the universally acknowledged mechanism, it was carefully estimated in this study. The results suggested that bromide anion directly participates in the acid hydrolysis reaction of MGP in a water solvent system and the participation of bromide anion is further pronounced in aqueous 74% and 82% 1,4-dioxane solvent systems. It was also suggested that chloride anion directly participates in these dioxane solvent systems.