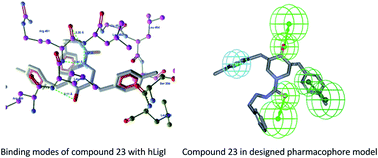
A pharmacophore model was generated and validated by using known human DNA ligase inhibitors for the identification of a novel series of monocarbonyl curcumin–thiourea/thiazole hybrids as human DNA ligase I (hLigI) inhibitors. These compounds (14–49) were synthesized and their antiligase and cytotoxic activities were evaluated in vitro. Several compounds from this series have shown significant inhibition of purified hLigI activity and exhibited a low micro molar range of cytotoxic activity against one or more cancer cell lines, with IC50 values ranging from 1.3–48.8 μM. Among these, compound 23 showed antiligase activity at an IC50 value 24.9 ± 1.8 μM, and selective cytotoxicity against DLD1 cancer cell line (IC50 value 8.7 ± 1.9 μM) compared to the reference curcumin (IC50 values were 51.9 ± 8.7 μM and 33.2 ± 1.8 μM for antiligase and cytotoxic activities against DLD1 cell line, respectively), and docking studies showed considerable interactions of compound 23 with hLigI. This new class of potent hLigI inhibitors will serve as a potential lead for further optimization and drug development.