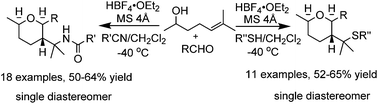
An efficient method has been developed for the synthesis of two new classes of tetrahydropyran derivatives comprising amide, tetrazole or benzothiazole moieties via a three-component reaction of 6-methylhept-5-en-2-ol, arylaldehydes and nitriles/thiols in the presence of a tetrafluoroboric acid diethyl ether complex. The reaction proceeds via the formation of an oxocarbenium ion. This protocol is highly diastereoselective and only single diastereomer has been isolated in each case.