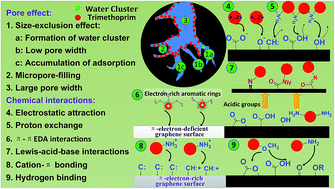
Five different types of activated carbon varying in porosity, structure, and functional groups were prepared and used as adsorbents. The effect of the key properties of each activated carbon on its adsorption capacity, rate and mechanisms on trimethoprim (TMP) removal were evaluated. The kinetics results suggested that chemical adsorption interactions and particle diffusion into micropores were the main rate-control steps for TMP adsorption, and the existence of mesopores promoted the diffusion of TMP into internal pores. The adsorption of TMP onto activated carbon can be attributed to the pore-filling effect (micropores and some narrow mesopores) and strong adsorptive interactions with the graphene surface or oxygenated groups. Regarding the surface area-normalized adsorption of TMP, porous activated carbon exhibited 50–500 times lower adsorption than nonporous carbon adsorbent due to the size-exclusion effect, especially when oxygen complexes presented on the edges of the pores of the activated carbon. From a system design point of view, a fast adsorption rate and high adsorption capacity are normally required, and these findings imply that activated carbon with high microporosity, a certain mesoporosity and approachable surface groups can have great application potential for TMP removal.