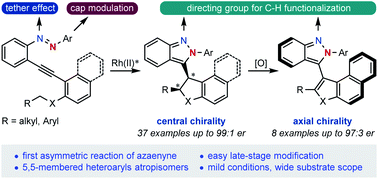
Described herein is a dirhodium(II)-catalyzed asymmetric cycloisomerization reaction of azaenyne through a cap-tether synergistic modulation strategy, which represents the first catalytic asymmetric cycloisomerization of azaenyne. This reaction is highly challenging because of its inherent strong background reaction leading to racemate formation and the high capability of coordination of the nitrogen atom resulting in catalyst deactivation. Varieties of centrally chiral isoindazole derivatives could be prepared in up to 99 : 1 d.r., 99 : 1 er and 99% yield and diverse enantiomerically enriched atropisomers bearing two five-membered heteroaryls have been accessed by using an oxidative central-to-axial chirality transfer strategy. The tethered nitrogen atom incorporated into the starting materials enabled easy late-modifications of the centrally and axially chiral products via C–H functionalizations, which further demonstrated the appealing synthetic utilities of this powerful asymmetric cyclization.