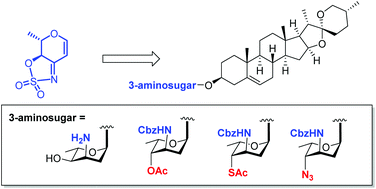
Attachment of various 3-aminodeoxy sugars to natural products or drugs is a prominent method for new drug development. However, access to structurally diversified 3-aminodeoxy sugars and glycosylation with aglycons are challenging. We synthesized a variety of unnatural 3-aminodeoxy sugars via diversified functionalization of a common glycal intermediate bearing a cyclic sulfamidate ketimine moiety. Based on these results, the α-selective glycosylation reactions of these 3-aminodeoxy sugars and the structural modification of diosgenin were further investigated.