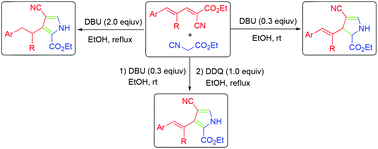
An efficient and divergent one-pot synthesis of 2,3-dihydro-1H-pyrroles, 3-alkyl-1H-pyrroles and 3-alkenyl-1H-pyrroles from readily accessible 2,4-pentadienenitriles with isocyanide based on reaction condition selection has been described. The reaction of 2,4-pentadienenitriles with ethyl isocyanoacetate undergoes a formal [2 + 3] annulation either to generate 2,3-dihydro-1H-pyrroles in the presence of DBU (0.3 equiv.) in EtOH at room temperature or to give 3-alkyl-1H-pyrroles in the presence of DBU (2.0 equiv.) in EtOH under reflux. Moreover, the 2,3-dihydro-1H-pyrroles could be converted to 3-alkenyl-1H-pyrroles with DDQ as an oxidant.