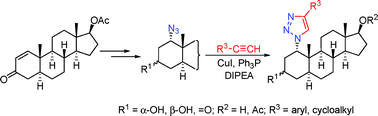
Stereoselective 1,4-Michael addition of azoimide to 17β-acetoxy-5α-adrost-1-en-3-one was carried out to furnish a 1α-azido-3-ketone, which was reduced to give the 3β- and 3α-hydroxy epimers in a ratio of 5 : 2. The Cu(I)-catalyzed 1,3-dipolar cycloaddition of the major isomer to terminal alkynes afforded 1α-triazolyl derivatives, which were deacetylated to the corresponding 3β,17β-diols or oxidized to the analogous 3-ketones. However, the ability of the minor 1α,3α-azidoalcohol to undergo similar cyclization was found to be affected significantly by the steric bulk of the substituents on the alkyne reaction partner. All triazolyl compounds were tested in vitro on three malignant gynecological cell lines (HeLa, MCF7 and A2780).