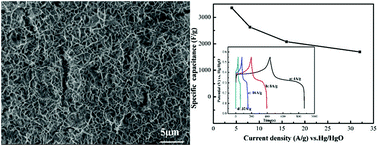
The effect of the electrodeposition temperature on the electrochemical performance of Ni(OH)2 electrode was investigated in this report. Ni(OH)2 was electrodeposited directly on nickel foam at different temperatures. The crystalline structure, morphology and specific surface area of the prepared Ni(OH)2 were characterized by X-ray powder diffraction (XRD), field emission scanning electronic microscopy (FESEM) and Brunauer–Emmett–Teller (BET). Electrochemical techniques such as cyclic voltammetry (CV), chronopotentiometry, and electrochemical impedance spectra (EIS) were carried out to systematically study the electrochemical performance of various Ni(OH)2 electrodes in 1 M KOH electrolyte. The results demonstrated that the electrodeposition temperature had obviously affected the properties of the Ni(OH)2. A pure α-Ni(OH)2 phase could be observed at low temperature. When the temperature increased to 65 °C, the β-Ni(OH)2 phase together with α-Ni(OH)2 phase were present. Moreover, the sample synthesized at 65 °C possessed a porous honeycomb-like microstructure and the corresponding specific capacitance was up to 3357 F g−1 at a charge–discharge current density of 4 A g−1, which suggested its potential application as an electrode material for supercapacitors.