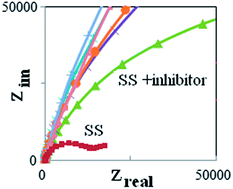
1-(4-nitrophenyl)-5-amino-1H-tetrazole was synthesized and its inhibiting action on the corrosion of 316L stainless steel (SS) in sulfuric acid was investigated by means of potentiodynamic polarization and electrochemical impedance spectroscopy (EIS). In the potentiodynamic polarization measurements the values of βc had small changes with increasing inhibitor concentration, which indicated that the 1-(4-nitrophenyl)-5-amino-1H-tetrazole was adsorbed on the metal surface and the addition of the inhibitor hindered the acid attack on the SS electrode. The shift in the anodic Tafel slope βa might be attributed to the modification of the anodic dissolution process due to the inhibitor module adsorption on the active sites. The results of the investigation show that the newly synthesized compound shows excellent inhibition efficiencies against the corrosion of SS in acidic solution. The adsorption of 1-(4-nitrophenyl)-5-amino-1H-tetrazole onto the SS surface followed the Langmuir adsorption model with the free energy of adsorption ΔG0ads of −9.44 kJ mol−1. Quantum chemical calculations were employed to give further insight into the mechanism of inhibition action of 1-(4-nitrophenyl)-5-amino-1H-tetrazole.