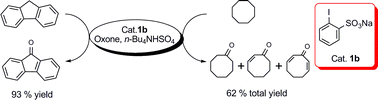
Catalytic oxidation of benzylic C–H bonds could be efficiently realized using IBS as a catalyst which was generated in situ from the oxidation of sodium 2-iodobenzenesulfonate (1b) by Oxone in the presence of a phase-transfer catalyst, tetra-n-butylammonium hydrogen sulfate, in anhydrous acetonitrile at 60 °C. Various alkylbenzenes, including toluenes and ethylbenzenes, several oxygen-containing functionalities substituted alkylbenzenes, and a cyclic benzyl ether could be efficiently oxidized. And, the same reagent system of cat. 1b/Oxone/cat. n-Bu4NHSO4 could be applied to the effective oxidation of alkanes as well.