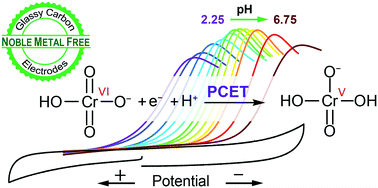
Electrochemical methods are an attractive option for the detection and reduction of toxic Cr(VI) to benign Cr(III) in drinking water. Here a method is reported for the reproducible detection and reduction of hexavalent chromium in water on glassy carbon electrodes, over a wide range of pH. This study allows for unmodified inexpensive carbon electrodes to join gold and gold modified electrodes as substrates for Cr(VI) removal from water.